Integrative Strategy of Testing Systems for Identification of Endocrine Disruptors Inducing Metabolic Disorders—An Introduction to the OBERON Project
Karine Audouze , Denis Sarigiannis , Paloma Alonso-Magdalena , Celine Brochot , Maribel Casas , Martine Vrijheid , Patrick J Babin , Spyros Karakitsios , Xavier Coumoul , Robert Barouki
Publication’s Abstract:
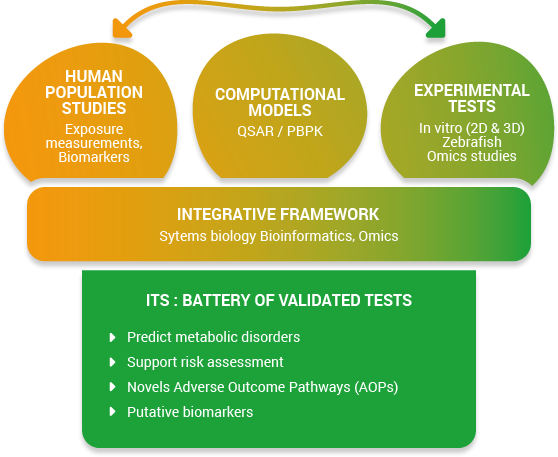
Exposure to chemical substances that can produce endocrine disrupting effects represents one of the most critical public health threats nowadays. In line with the regulatory framework implemented within the European Union (EU) to reduce the levels of endocrine disruptors (EDs) for consumers, new and effective methods for ED testing are needed. The OBERON project will build an integrated testing strategy (ITS) to detect ED-related metabolic disorders by developing, improving and validating a battery of test systems. It will be based on the concept of an integrated approach for testing and assessment (IATA). OBERON will combine (1) experimental methods (in vitro, e.g., using 2D and 3D human-derived cells and tissues, and in vivo, i.e., using zebrafish at different stages), (2) high throughput omics technologies, (3) epidemiology and human biomonitoring studies and (4) advanced computational models (in silico and systems biology) on functional endpoints related to metabolism. Such interdisciplinary framework will help in deciphering EDs based on a mechanistic understanding of toxicity by providing and making available more effective alternative test methods relevant for human health that are in line with regulatory needs. Data generated in OBERON will also allow the development of novel adverse outcome pathways (AOPs). The assays will be pre-validated in order to select the test systems that will show acceptable performance in terms of relevance for the second step of the validation process, i.e., the inter-laboratory validation as ring tests. Therefore, the aim of the OBERON project is to support the organization for economic co-operation and development (OECD) conceptual framework for testing and assessment of single and/or mixture of EDs by developing specific assays not covered by the current tests, and to propose an IATA for ED-related metabolic disorder detection, which will be submitted to the Joint Research Center (JRC) and OECD community.


 Download presentation leaflet
Download presentation leaflet